In addition to these notes, a nice intro can be found
here.
The Nucleus
- The nucleus consists of protons and neutrons.
- The number of protons is known as the atomic number and is generally
designated by the letter Z.
- The total number of nucleons (protons or neutrons) is known as mass
number and is generally designated by the letter A.
- Symbolically, A=Z+N, where N is the number of neutrons.
Radioactive decay
When an atomic nucleus is unstable, decay brings the nucleus to a more stable
state.
There are 5 basic types of radioactive decay,
although a given nucleus may decay through a combination of these:
- Alpha (α) emission--reduces mass number
by 4 and atomic (proton) number by 2
- alpha particle is actually a helium nucleus
- most common type of decay.
- least penetrating; can be stopped by a sheet of paper
- smoke detectors use the alpha radiation from americium-241 to detect
smoke particles in the air.
- Example: 238/92 U --> 234/90 Th + 4/2 He (note that 234+4=238 and
90+2=92)
- Beta (β) emission--increases atomic (proton) number
by 1
- beta particle is actually an electron which originates in the
nucleus
- can penetrate skin
- Gamma (γ) emission--does not change nuclear composition
- gamma radiation is a form of electromagnetic radiation
- most penetrating, will go through flesh and bone
- Electron Capture--reduces atomic (proton) number by 1
- Positron Emission--reduces atomic (proton) number by 1
- positron is essentially an electron with a positive charge

Stability
- Of the 7000 or so nuclides that might possibly exist, about 2000 have
either been found in nature or created in the laboratory, and of those only
256 are stable and do not undergo radioactive decay.
- Uranium-238 requires 8 alpha decays and 6 beta decays to eventually
become Lead-206, a stable element.
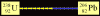
Radiation dosage
- Radiation doses are measured in rads (short for radiation absorbed
dose), where 1 rad corresponds to 0.01 joule of energy deposited per
kilogram of tissue.
- Because alpha, beta, and gamma radiation differ in penetrating and
ionizing ability, both the energy dose of the radiation and its
effectiveness in causing human tissue damage must be considered.
- The rem (short for roentgen equivalent for man), which takes into
account both the dosage and its relative biological effectiveness, is
therefore the unit that we generally use when discussing the biological
effects of radiation. The SI unit of radiation biological effectiveness is
the sievert (Sv), where 1 Sv = 100 rem.
- The curie is a unit of activity of radioactive substances equivalent to
3.70 × 1010 disintegrations per second, or approximately the
amount of activity produced by 1 g of radium-226.
- The average U.S. resident is exposed to about 0.2 rem of radiation each
year, of which about 82% derives from natural
sources and 18% is related to human activities.
Sources of radiation
- The largest single source of radiation exposure to the general public is
naturally occurring radon gas, which comprises approximately 55% of the
annual background dose.
- Radon is a decay product of radium whose own origin traces back to the
decay of Uranium. Uranium is found in many common rocks, notably granite.
- Radon is the second most common cause of lung cancer, after cigarette
smoking, and radon-induced lung cancer is thought to be the 6th leading
cause of cancer death overall. In the US, Radon claims about 20,000 lives annually.

Return to class notes TOC.
Page last modified:
