Layers and composition
- The Earth's atmosphere is a VERY THIN layer of gas that surrounds the planet.
- It blocks out dangerous short-wavelength radiation (e.g., UV, x-rays, gamma
rays, etc.) from the sun
- It provides temperature regulation for the planet.
- It provides the gases that living organisms need for life.
- Atmosphere layers:
- The troposphere
- first layer above the surface
- contains half of the Earth’s atmosphere
- The stratosphere
- jets fly in the stratosphere because it is very stable
- ozone layer absorbs harmful rays from the Sun here
- The mesosphere
- meteors burn up in the mesosphere
- The thermosphere
- layer with auroras
- where the space shuttle orbits
- Composition
- Nitrogen (78%)
- Oxygen (21%)
- Other gases (1%)

Ozone
- For nearly a billion years, ozone molecules in the atmosphere have
protected life on Earth from the harmful effects of ultraviolet rays.
- Human exposure to UV radiation increases the risk of skin cancer,
cataracts, and a suppressed immune system. UV-B exposure can also damage
terrestrial plant life, single cell organisms, and aquatic ecosystems.
- The majority of these ozone molecules resides in a
layer between 10 and 40 kilometers (6 and 25
miles) above the Earth's surface in the stratosphere.
- In the past 60 years or so, human activity has contributed to the
deterioration of the ozone layer. One of the biggest culprits are man-made
chlorines, primarily chloroflourobcarbons (CFCs).
- Each spring in the stratosphere over Antarctica (Spring in the southern
hemisphere is from September through November.), atmospheric ozone is
rapidly destroyed by chemical processes. Over the course of 2-3 months,
approximately 50% of the total column amount of ozone in the atmosphere
disappears. At some levels, the losses approach 90%. This has come to be
called the Antarctic ozone hole.
- In spring, temperatures begin to rise, the ice evaporates, and the ozone
layer starts to recover.
- The ozone "hole" is really a reduction in concentrations of ozone high
above the earth in the stratosphere. The ozone hole is defined
geographically as the area wherein the total ozone amount is less than 220
Dobson Units. The ozone hole has steadily grown in size (up to 27 million
sq. km.) and length of existence (from August through early December) over
the past two decades.
- After a series of rigorous meetings and negotiations, the Montreal
Protocol on Substances that Deplete the Ozone Layer was finally agreed upon
on September 16, 1987 at the Headquarters of the International Civil
Aviation Organization in Montreal.
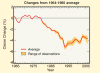
- Satellite observations show a decrease in global
total ozone values over more than two decades. The graph above compares
global ozone values (annual averages) with the average from the period 1964
to 1980. Seasonal and solar effects have been removed from the data. On
average, global ozone decreased each year between 1980 and the early 1990s.
The decrease worsened during the few years when volcanic aerosol from the
Mt. Pinatubo eruption in 1991 remained in the stratosphere. Now global ozone
is about 4% below the 1964- to-1980 average.
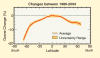
- The graph below compares ozone changes for
different latitudes during the years 1980-2004. The largest decreases
have occurred at the highest latitudes in both hemispheres because of the
large winter/spring depletion in polar regions. The losses in the Southern
Hemisphere are greater than those in the Northern Hemisphere because of the
Antarctic ozone hole. Long-term changes in the tropics are much smaller
because reactive halogen gases are less abundant in the tropical lower
stratosphere.

FYI: If all the water vapor in the atmosphere were condensed, the sea level
would rise by only about 2.5 cm.
FYI: Without the greenhouse effect, the Earth's average surface temperature
would be 0° F, not 57.9° F.
Global Warming
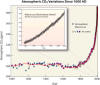
- Atmospheric carbon dioxide levels have risen
steadily since the 1800s and the upward trend appears to be accelerating.
Most scientists attribute this dramatic increase to human industrial
activities, primarily fossil fuel burning and land use changes (e.g.,
deforestation).
- Our output of roughly 29 gigatons of CO2 constitutes roughly 5% of the
750 gigatons moving through the carbon
cycle each year. This amount adds up because the land and ocean cannot
absorb all of the extra CO2. In fact, only about 40% of this
additional CO2 is absorbed. The rest remains in the atmosphere, and as a
consequence, atmospheric CO2 is at its highest level in 15-20 million years
(Tripati
2009). (A natural change of 100 ppm normally takes 5,000-20,000 years.
The recent increase of 100 ppm has taken just 120 years).
- The measurements of CO2 are from two sources: air trapped inside ice cores, and
direct measurements of the atmosphere (taken from the Hawaiian peak Mauna
Loa) since the late 1950s. (Figure courtesy of
Scott Doney, Woods Hole Oceanographic Institution)
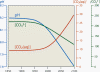
- The rise in atmospheric carbon dioxide appears to correlate with a rise
in average global temperature and a rise in ocean
acidity.

Return to class notes TOC.
Page last modified:
